MMP-13 antagonist | BI-4394
Highlights
BI-4394 is a highly potent inhibitor of matrix metalloproteinase MMP-13 (IC50 = 1 nM) with excellent selectivity (> 1,000-fold) against several other matrix metalloproteinases. This compound is a high-quality tool to test, in vitro, biological hypothesis involving this target.
According to the UK Third Generation Cannabinoid Act, the molecule cannot be shipped to the United Kingdom.

Background information
Target Information
Matrix metalloproteinases (MMPs) are zinc- and calcium-dependent peptidases, involved in the cleavage of collagen, gelatin and other proteins in the extracellular matrix and tissue remodelling. There are approximately 23 known human MMPs that are grouped into subtypes based on their substrates. MMPs have a conserved active site motif where a tris(histidine)-bound zinc (II) acts as the catalytic site for substrate hydrolysis. MMP-13 (also known as collagenase 3, CLG3) is the most efficient enzyme of this class at degrading collagen II, the committed step in articular cartilage degradation and progressive joint damage associated with rheumatoid arthritis (RA). Broad-spectrum MMP inhibitors have failed in clinical trials at least in part due to a joint-stiffening side effect, termed musculoskeletal syndrome (MSS). This was likely due to inhibition of MMPs other than MMP-13 and high selectivity for MMP-13 over other MMPs is therefore favourable.
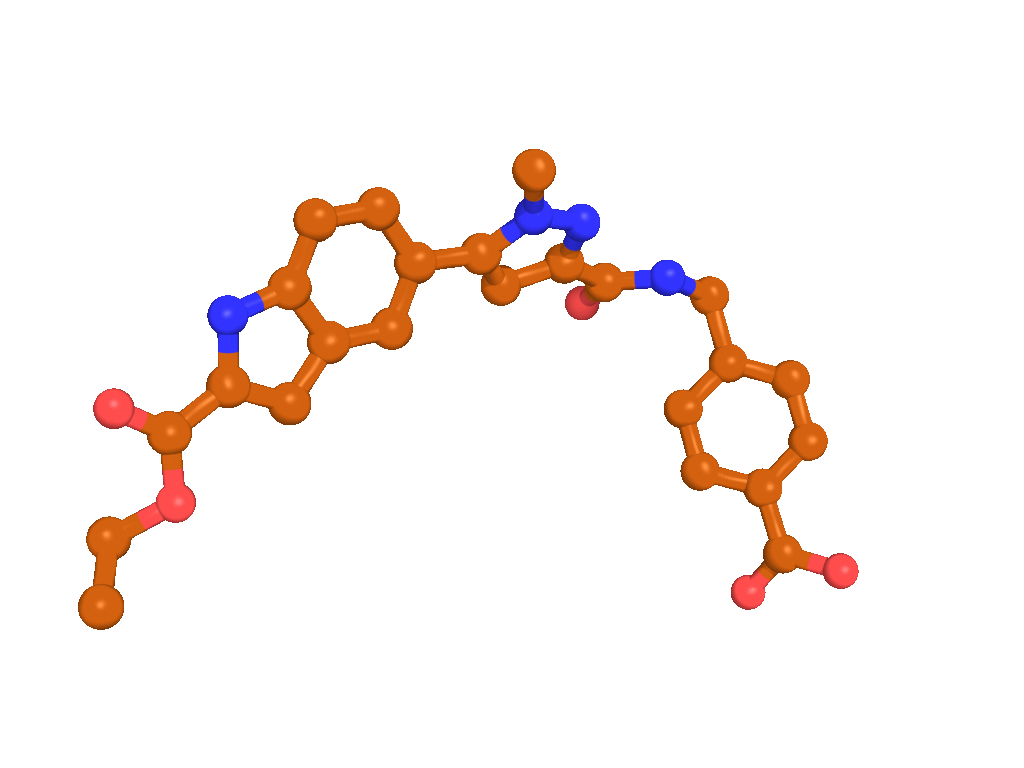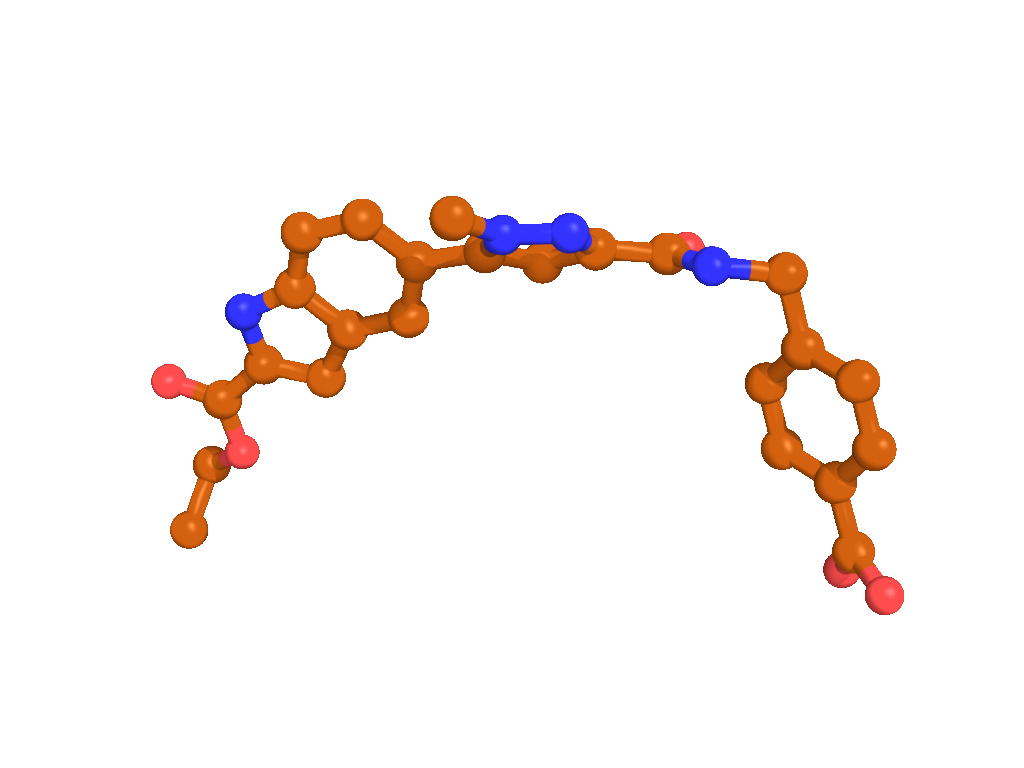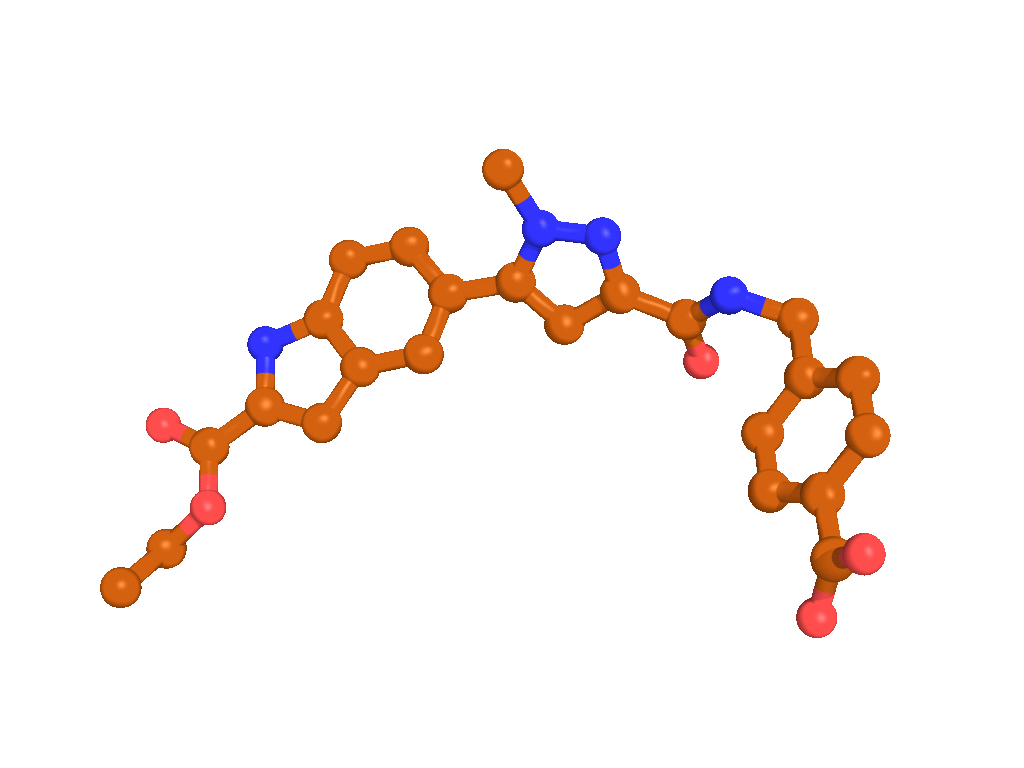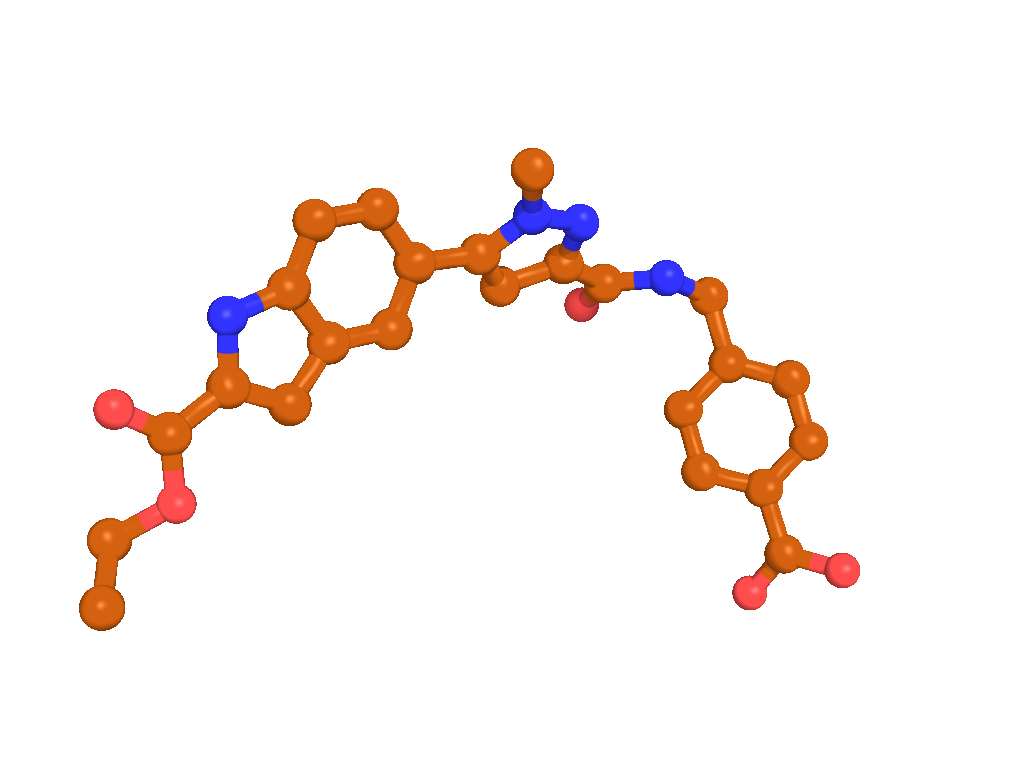
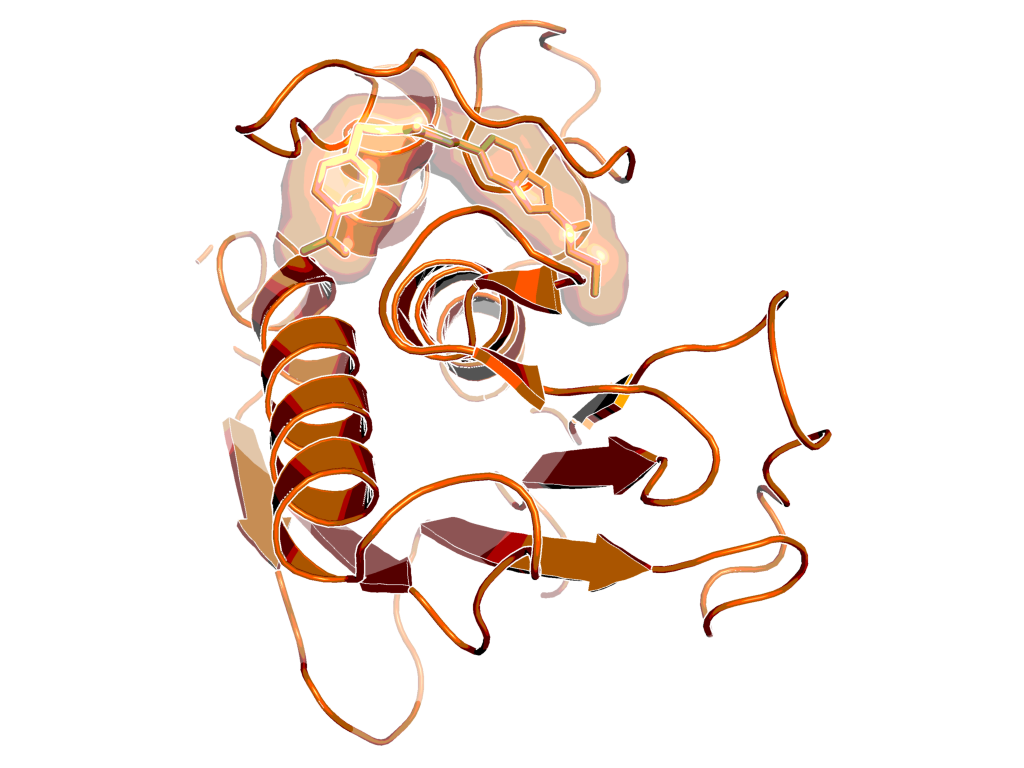
BI-4394 bound to MMP-13, as observed by X-ray crystallography (PDB code: 5BPA)
In vitro activity
BI-4394 is a potent inhibitor of MMP-13 with an IC50 value of 1 nM.
| Probe name / negative control | BI-4394 | BI-4395 |
| MW [Da] | 446.5 | 374.4 |
| Inhibition of MMP-13 (IC50) [nM] | 1 | >26,000 |
| Inhibition of bovine nasal cartilage with human full length MMP-13 (IC50) [nM] | 31 | n.d. |
In vitro DMPK and CMC parameters
| Probe name / negative control | BI-4394 | BI-4395 | ||
| logP (pH 2) | 1.9 | n.d. | ||
| Solubility @ pH 7.4 [µg/ml] | 60 | >96 (pH 7) | ||
| Solubility @ pH 4 [µg/ml] | <0.1 | <0.1 | ||
| CACO permeability @ pH 7.4 [*10-6 cm/s] | 0.6 | n.d. | ||
| CACO efflux ratio | 27 | n.d. | ||
| Microsomal stability (human/rat) [% QH] | 40 / 41 | 25 / n.d. | ||
| Plasma protein binding (human) [%] | 98 | n.d. | ||
In vivo DMPK parameters
| BI-4394 | RAT |
| Clearance [ml/(min*kg)]b | 39 |
| Mean residence time after i.v. dose [h] | 0.5 |
| F [%] | 39 |
| Vss [l/kg] | 0.4 |
b i.v. dose: 1 mg/kg
Negative control

BI-4395 which serves as a negative control
Selectivity
BI-4394 is highly (> 1000 fold) selective against other matrix metalloproteinases (MMP-1, 2, 3, 7, 8, 9, 10, 12, 14):
MMP | 1 | 2 | 3 | 7 | 8 | 9 | 10 | 12 | 13 | 14 |
IC50 [µM] | >22 | 18 | >22 | >22 | >22 | 8.9 | 16 | >22 | 0.001 | 8.3 |
| SELECTIVITY DATA AVILABLE | BI-4394 | BI-4395 |
SafetyScreen44™ with kind support of  | Yes | Yes |
| Invitrogen® | Yes | No |
| DiscoverX® | No | No |
| Dundee | No | No |
Invitrogen:
18/56 kinases hit >50 inhibition at 10 µM: STK6 (99%), MAPKAPK2 (99%), RPS6KA3 (95%), MAPK14 (94%), GSK3B (94%), AMPK A1B1G1 (92%), PRKACA (90%), PIM1 (86%), KDR (83%), AKT1 (76%), SRC (75%), DYRK3 (72%), MAP4K4 (68%), MET (57%), JAK3 (56%), IKBKB (52%), ABL1 (52%), NEK1 (51%).
Download selectivity data:
BI-4394_selectivityData_0.xlsx
BI-4395_selectivityData.xlsx
Co-crystal structure of the BI probe compound and the target protein
X-Ray co-crystal structure of BI-4394 bound to MMP-13 is available (see Figure "BI-4394 bound to MMP-13, as observed by X-ray crystallography (PDB code: 5BPA)", PDB code: 5BPA).
Summary
BI-4394 is a potent and highly selective inhibitor of MMP-13 that can be used as tool compound to test biological hypotheses in vitro.
According to the UK Third Generation Cannabinoid Act, the molecule cannot be shipped to the United Kingdom.
Supplementary data
2 D structure formats available
MMP-13 antagonist ǀ BI-4394.png
MMP-13 antagonist ǀ BI-4394.smiles
MMP-13 antagonist ǀ BI-4394.sdf
Negative control | BI-4395.png
References
Fragment-Based Discovery of Indole Inhibitors of Matrix Metalloproteinase-13
Taylor S. J., Abeywardane A., Liang S., Muegge I., Padyana A. K., Xiong Z., Hill-Drzewi M., Farmer B., Li X., Collins B., Li J. X., Heim-Riether A., Proudfoot J., Zhang Q., Goldberg D., Zuvela-Jelaska L., Zaher H., Li J., Farrow N. A.
J. Med. Chem. 2011, 54, 8174.
Improving potency and selectivity of a new class of non-Zn-chelating MMP-13 inhibitors
Heim-Riether A, Taylor S. J., Liang S., Gao D. A., Xiong Z., Michael August E., Collins B. K., Farmer B. T. 2nd, Haverty K., Hill-Drzewi M., Junker H. D., Mariana Margarit S., Moss N., Neumann T., Proudfoot J. R., Keenan L. S., Sekul R., Zhang Q., Li J., Farrow N. A.
Bioorg. Med. Chem. Lett. 2009, 19, 5321.
SAR studies of non-zinc-chelating MMP-13 inhibitors: Improving selectivity and metabolic stability
Gao D.A., Xiong Z., Heim-Riether A., Amodeo L., August E. M., Cao X., Ciccarelli L., Collins B. K., Harrington K., Haverty K., Hill-Drzewi M., Li X., Liang S., Margarit S.M., Moss N., Nagaraja N., Proudfoot J., Roman R., Schlyer S., Keenan L.S., Taylor S., Wellenzohn B., Wiedenmayer D., Li J., Farrow N. A.
Bioorg. Med. Chem. Lett. 2010, 20, 5039.
Discovery of N-(4-Fluoro-3-methoxybenzyl)-6-(2-(((2S,5R)- 5-(hydroxymethyl)-1,4-dioxan-2-yl)methyl)-2H-tetrazol-5-yl)-2-methylpyrimidine-4-carboxamide. A Highly Selective and Orally Bioavailable Matrix Metalloproteinase-13 Inhibitor for the Potential Trea
Ruminski P. G., Massa M., Strohbach J., Hanau C. E., Schmidt M., Scholten J. A., Fletcher T. R., Hamper B. C., Carroll J. N., Shieh H. S., Caspers N., Collins B., Grapperhaus M., Palmquist K. E., Collins J., Baldus J. E., Hitchcock J., Kleine H. P., Rogers M. D., McDonald J., Munie G. E., Messing D. M., Portolan S., Whiteley L. O., Sunyer T., Schnute M. E.
J. Med. Chem. 2016, 59, 313.
How to cite opnMe?
When you plan a publication, please use the following acknowledgement:
BI-4394 was kindly provided by Boehringer Ingelheim via its open innovation platform opnMe, available at https://opnme.com.
Papers with our molecules. Published by you.
On-demand release of a selective MMP-13 blocker from an enzyme-responsive injectable hydrogel protects cartilage from degenerative progression in osteoarthritis
Roy H. S., Murugesan P., Kulkarni C., Arora M., Nagar G. K., Guha R., Chattopadhyay N., Ghosh D.
J Mater Chem B. 2024, 12(22):5325-5338.






























